|

VOLTA'S
PILE - copper and zinc plates with saline soaked cloth separators.
A battery is basically a device for making electrically charged atoms—known as ions—travel from one point to another. When electrical charges move, they create an electric current. This current powers anything connected to the battery.
Almost everything in battery design comes down to the materials of which the anode, cathode, and electrolyte are made. They determine how many ions the battery can store and how fast it can pump them out.
The
invention of batteries did not change the world overnight, nor did the
inventor realise what he'd opened the door to. The voltaic pile was the first electric
battery that could continuously provide an electric current to a circuit. It was invented by Italian physicist Alessandro Volta, who published his experiments in 1799.
The voltaic pile enabled a rapid series of discoveries including the electrical decomposition (electrolysis) of
water into oxygen and
hydrogen by William Nicholson and Anthony Carlisle (1800), so starting the science of
electrochemistry. The
modern definition of a battery is a device that stores energy and provides electricity
on demand. Conventional thinking goes out of the window with ammonia,
hydrogen and methanol, now able to provide power in a similar package, with
the advent of compatible fuel cells. These are hybrids. CONVENTIONAL:
PRIMARY & SECONDARY CELL BATTERIES
Conventional batteries fall into two categories: primary and secondary cells. Primary cell batteries are non rechargeable. They will be exhausted after their component parts are used up (converted). They may also be referred to as dry cell
batteries, where the electrolytes are commonly paste.
Secondary cell batteries (or accumulators) can recharged repeatedly after each discharge, by passing current through them in reverse direction. This replenishes the chemicals that were used up during discharge. By this means
electricity generated from renewables (solar and wind) might be stored. 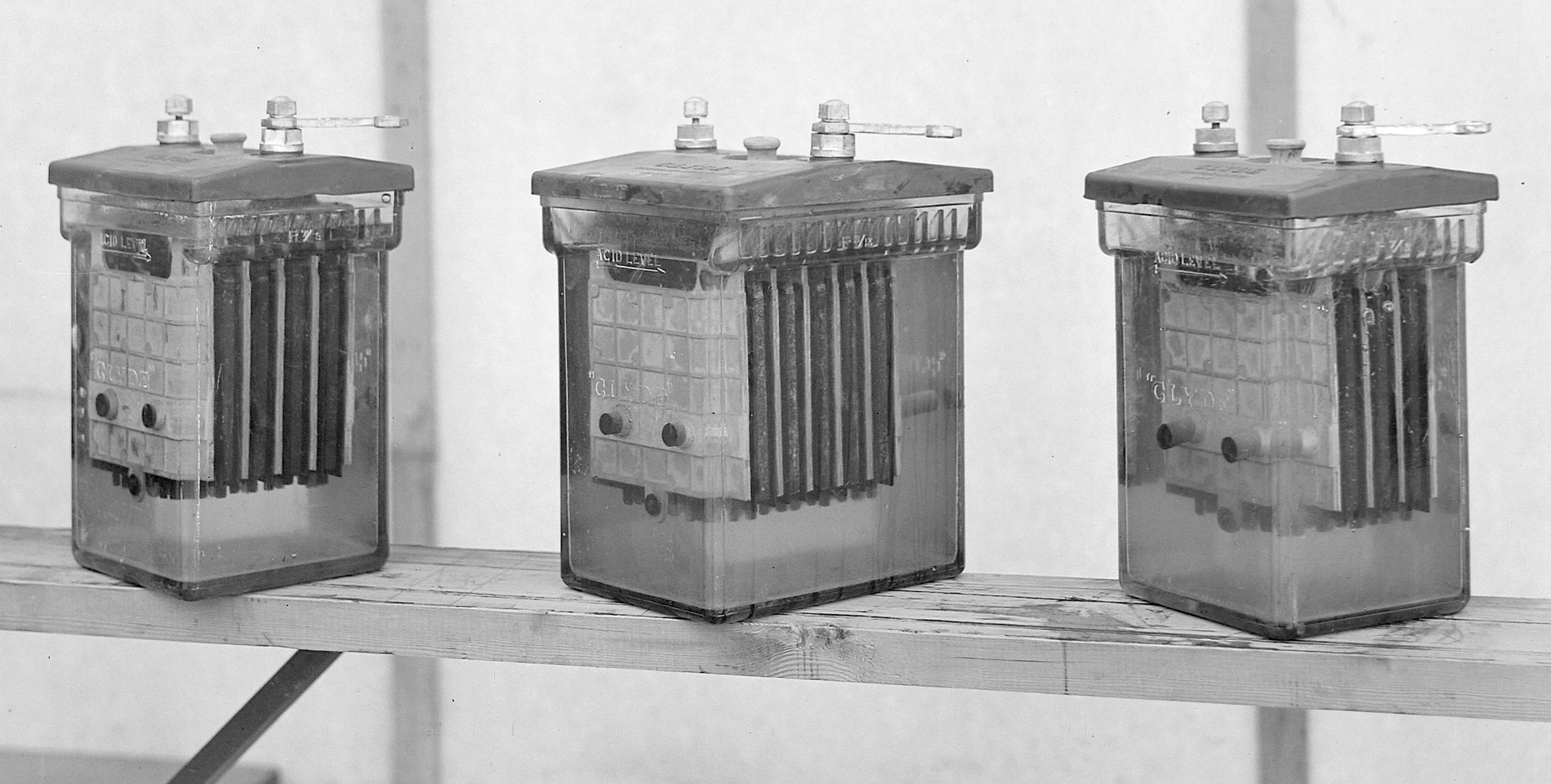
LEAD-ACID -
Banks of lead acid batteries were used to store generated electricity, for
lighting and telephone exchanges. Only the wealthy could afford the cells
and charging equipment, until power was supplied via cables underground and
overhead, to form a grid
network. Very early supplies were direct current, later replaced by
alternating current to allow transforming, and transmission at high voltages
to reduce resistance losses in the cables.
THE FIRST ACCUMULATOR (RECHARGEABLE BATTERY)
The lead–acid battery was invented in 1859 by French physicist Gaston Planté and is the earliest type of rechargeable battery. This followed the French scientist Nicolas Gautherot's observation in 1801, that wires that had been used for electrolysis experiments would themselves provide a small amount of "secondary" current after the main battery had been disconnected.
Planté's lead–acid battery was the first battery that could be recharged by passing a reverse current through it. Planté's first model consisted of two lead sheets separated by rubber strips and rolled into a spiral.
Despite having a very low energy-to-weight ratio and a low energy-to-volume ratio (energy density), the ability of the lead-acid battery to supply high surge currents means that the cells have a relatively large power-to-weight ratio. These features, and the low cost, make them ideal for use in
ICE automobiles to provide the high current to turn a starter motor. Larger
lead-acid batteries were used in submarines and in rural locations to store
electricity in between charging cycles. 
GASTON
PLANTE - The lead-acid batteries of the French inventor were used to
store electricity for owners of larger houses with generators, who wanted
light bulbs instead of gas lamps or candles. The last surviving building
equipped with a large battery store, is thought to be in Herstmonceux,
Sussex, England. This building ran a gas powered generator by day to charge
batteries and store enough energy for the nearby village C. 1900. By 1911
the generating works of Charles de Roemer had established a client base and
was supplying electricity to a bakery. By 1913 Captain de Roemer was giving
cooking demonstrations at the then village hall (now demolished).
THE LECLANCHÉ CELL
In 1866, Georges Leclanché invented a (primary) battery that consisted of a zinc anode and a manganese dioxide cathode wrapped in a porous material, dipped in a jar of ammonium chloride solution. The manganese dioxide cathode had a little carbon mixed into it as well, which improved conductivity and absorption. It provided a voltage of 1.4 volts. This cell achieved very quick success in telegraphy, signaling and electric bell work.
A dry cell is a type of electric battery, commonly used for portable electrical devices. It was developed in 1886 by the German scientist Carl Gassner. The modern version was developed by Japanese Yai Sakizo in 1887.
Unfortunately, primary cells do not fit well into any sustainable scenario
at present. 
ELECTRICITY
GENERATION Michael Faraday discovered
electromagnetic induction in 1831. Building on the experiments of Franklin and others, he observed that he could create or “induce” electric current by moving magnets inside coils of copper wire. The discovery of electromagnetic induction (power generation) revolutionized how we use energy. The Scottish physicist James Clerk Maxwell developed his profound mathematical electromagnetic theory from 1855 onward.
Enter Thomas Edison with his electric light bulb in the 1880s and small generators in basements for wealthy homes, because there was no grid. J. P Morgan funded Pearl Street, in New York and Edison's underground copper cables to create the first power supply grid, heralding the birth of the power supply industry.
Then Samuel Insull came into the frame to develop a business model for a commodity that had to be consumed the moment it was produced, where battery storage was expensive. Insull achieved economies of scale for larger more efficient generators made by General Electric. He also used high-voltage power lines to supply the suburbs and
countryside by 1903.
With consolidation, mass production and consumption, rural electrification and two-part pricing from networked
power, Samuel Insull did for electricity what Henry Ford did for the automobile. He turned a luxury product into an affordable part of everyday life for millions of Americans.
That model spread all over the civilised world with coal
and oils as the fuels that choked the planet with sulfurous smogs, to give
us global warming. And so we are looking for clean air solutions to keep on
enjoying electricity for modern living. But
with electricity generation from wind
turbines and solar
panels being nature dependent, and out of kilt with people consumption,
we again need to crack the storage problem, with hydrogen gas and fuels
cells, and lithium batteries providing possible solutions, where Planté's
lead-acid batteries are well and truly out of the frame. 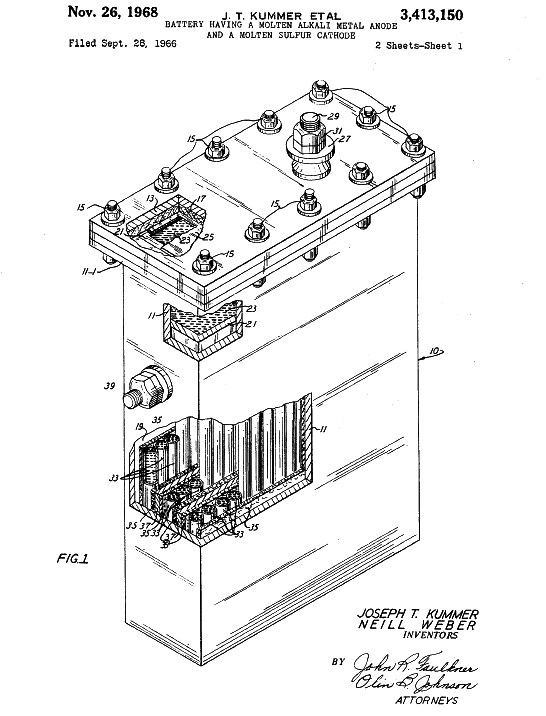
FORD MOLTEN METAL BATTERY - In 1966, Ford filed a patent for a molten
metal sulfur battery, as depicted above. In 1976, Exxon filed a patent application for a lithium-based
battery that operated at room temperature, but was unstable, where the Exxon formula set batteries on fire.
In 1980, John Goodenough and a team of scientists at Oxford University,
cracked the lithium cobalt formula, on which modern car batteries are based.
But the hunt continues for a super battery - and probably always will.
THE GUARDIAN 3 JANUARY 2021 - UK CAR MAKERS HAVE 3 YEARS TO SOURCE LOCAL EV BATTERIES
UK carmakers face a three-year scramble to source electric car batteries locally or from the EU to avoid tariffs on exports following the Brexit free trade deal, according to industry analysts.
The Christmas Eve deal means that all UK-EU trade in cars and parts will continue to be free of tariffs or quotas after the the Brexit transition period ended on Friday, as long as they contain enough content from either UK or EU factories. The deal came as a major relief to the embattled car industry.
Batteries will at first be allowed to contain up to 70% of materials from countries outside the EU or the UK. However, from 1 January 2024 that requirement will tighten to 50%. This will mean that sourcing battery materials from within the UK or EU will be the only realistic option for UK carmakers to avoid EU tariffs from 2024 onwards, according to Alessandro Marongiu, a trade analyst at the lobby group the Society of Motor Manufacturers and Traders (SMMT).
The rules mean it will be vital for UK carmakers to secure a battery supply from the the EU or the UK, said Mike Hawes, the SMMT’s chief executive. The deal makes it “imperative that the UK secures at pace investment in battery gigafactories and electrified supply chains”, he said.
Ian Henry, whose AutoAnalysis consultancy works with a number of major carmakers, said one key issue in preserving the UK car industry was creating an entire supply chain, including manufacturing important and chemically complicated parts such as the cathodes crucial to lithium-ion batteries.
“By the mid-2020s, the UK has got to be doing a lot more than just assembling bits from a kit,” said Henry. “Several core processes in battery manufacturing will have to take place here in order for the UK to have a viable electric vehicle industry.”
The vast majority of batteries used in UK and EU electric cars are sourced from east Asian companies such as China’s CATL, Korea’s LG Chem or Japan’s Panasonic. However, European carmakers and governments are pouring
billions of
euros into new battery plants, with at least 10 credible efforts under way from companies including Sweden’s Northvolt and the French oil major Total, according to data company LMC Automotive.
The UK is lagging behind, with no fully funded plans to begin battery production in Britain, despite the promise of government funding and industry support for British “gigafactories” capable of large-scale battery production. The government-backed Faraday Institution this year said a failure to build a UK battery supply chain could cost more than 100,000 jobs by 2040.
The Brexit deal was welcomed by the startup Britishvolt, which is the only company with public plans to build a gigafactory in the UK. Britishvolt last month bought rights to a site in Blyth, Northumberland, where it plans to build a factory, subject to
fundraising and a planning application for enlargement and change of use.
The Brexit deal’s provisions fit perfectly with Britishvolt’s ambitious plans to start battery production at scale by the end of 2023. It hopes to start building the plant in the summer. A spokesman said the deal would allow it to serve both the UK’s domestic automotive industry and carmakers in
Europe. 
FEBRUARY
2015 - At 92, John Goodenough frequented his smallish office every day at the University of Texas at Austin. That, he says, is because he’s not finished. Thirty-five years after his blockbuster, the electric car still can’t compete with the
internal combustion engine on price. When solar and wind power produce electricity, it must be either used immediately or lost forever—there is no economic stationary battery in which to store the power. Meanwhile, storm clouds are gathering: Oil is again cheap but, like all cyclical commodities, its price will go back up. The climate is warming and becoming generally more turbulent.
John Goodenough grew up in a sprawling home near New Haven, Connecticut, where his father, Erwin, was a scholar on the history of religion at Yale. When John was 12, he was sent on scholarship at Groton, a private boarding school in Massachusetts.
After World War II, Goodenough, as a 24-year-old Army captain posted in the Azores Archipelago off the coast of Portugal, received a telex ordering him to Washington, DC. Educators had stumbled on unspent budget money and advocated using it to send 21 returning Army officers through graduate studies in physics and math.
So he found himself at the University of Chicago, studying under some of the leading physicists of the era, including Edward Teller and Enrico Fermi. As Goodenough registered for preliminary undergraduate classes, necessary to catch up with the others, a professor remarked, “I don’t understand you veterans. Don’t you know that anyone who has ever done anything significant in physics has already done it by the time he was your age?”
But it turned out that Goodenough had an intuition for physics. After obtaining his doctorate in 1952, he went to work at MIT’s Lincoln Laboratory, which the US Air Force had funded the year before to create the country’s first air defense system, known as SAGE.
By the mid-1970s, Goodenough was fixated on finding a scientific answer to the OPEC-led energy crisis, which seemed to be the largest problem facing the country.
A friend sent word of an opportunity across the Atlantic. Oxford University required a professor to teach and manage its inorganic chemistry lab. In 1976, Goodenough was surprised to be selected; he was not a chemist and had completed just two college-level chemistry courses.

Goodenough was a tough professor. Clare Grey, a student of his at Oxford, recalled a physics course that started with 165 students. After a stern Goodenough lecture, she was one of just eight to return for the second class.
Goodenough directed two postdoctoral assistants to methodically work their way through structures containing a group of oxides, to find out at what voltage lithium could be extracted from the oxides - and how much lithium could be pulled out of the atomic structure.
Their answer was that about half of the lithium could be pulled from the cathode at 4 volts before it crumpled. That was plenty for a powerful, rechargeable battery. Of the oxides they tested, the postdocs found that cobalt was the best and most stable for the purpose.
Completed in 1980, four years after Goodenough arrived at Oxford, the lithium-cobalt-oxide cathode was a breakthrough even bigger than Ford’s sodium-sulfur configuration. It was the first lithium-ion cathode with the capacity, when installed in a battery, to power both compact and relatively large devices.
In 1991, Sony combined Goodenough’s cathode and a carbon anode into the world’s first commercial rechargeable lithium-ion battery. The result was an overnight blockbuster. Despite his central role in developing this technology, Goodenough earned no royalties. Oxford had declined to
patent Goodenough’s
cathode - the university seemed to see no advantage in owning intellectual property.
In 1883, Thomas
Edison, misled too many times in the midst of creating his electronic empire, sized up rechargeable batteries as a mere fable. He wrote:
"The storage battery is, in my opinion, a catchpenny, a sensation, a mechanism for swindling the public by stock companies. The storage battery is one of those peculiar things which appeals to the imagination, and no more perfect thing could be desired by stock swindlers than that very selfsame thing. … Just as soon as a man gets working on the secondary battery it brings out his latent capacity for lying."
Warren Buffett spent $230 million to buy 10% of BYD, a Chinese car company that announced a new lithium-iron-phosphate-powered electric car. No one spoke of the source of BYD’s batteries.
In 2009, A123 sold shares in an initial public offering. Chiang’s charisma, the MIT name, and the general tenor of the times created an aura of sizzle, and the share price surged by 50% on the first day of trading. Chiang’s company raised $587 million, the biggest IPO of the year and a tremendous payday for him and all involved. Except, again, Goodenough.
In the end, the University of Texas settled with NTT. The payoff to the school was $30 million along with a share of any profit from its Japanese patents. NTT admitted no wrongdoing. Goodenough received nothing from A123. One might see a certain poetic justice in what has happened since. In 2012—just three years after the IPO—A123 ended up in bankruptcy.
In 2013, Goodenough received the National Medal of Science from president Barack
Obama, and in 2009 the Enrico Fermi Award.
In 2015 Goodenough was passionate about ending his career with a big invention. He was trying to make a super-battery, one that will make electric cars truly competitive with combustion, and also economically store wind and solar power.
HYBRIDS:
AMMONIA, HYDROGEN & METHANOL BATTERIES The
UK, or any country in Europe or anywhere in the world for that matter, could
be a producer of hydrogen batteries,
that do the same job as lithium batteries, but provide a longer range
between refuelling. Though this would be subject to licensing arrangements
for compatible designs. Of course meaning filling up with hydrogen, or, and
especially for robotic vehicles, using an automatic vending system such as SmartNet™
where humans are not needed to keep robotaxis,
shuttles and robotrucks
operational, provided they run on electricity and have a compatibility kit
bolted on. The
same format for hydrogen gas or liquid
fuels, might be adopted for ammonia
and methanol.
Even hydrogen peroxide is a contender. 
HOME
TO ROOST - Dr John Goodenough invented the lithium-cobalt battery in
1980 at Oxford University, now these batteries are mostly produced in Asia.
Forty years on and lithium batteries for automobiles may go into production in the
UK. All the while Toyota is pushing for
solid state batteries with millions of dollars of state aid, encouraging
R&D, just in case of another breakthrough. We either need another
breakthrough, or switch over to cartridge exchange. Porsche invented
cartridge exchange for his electric vehicles,
LINKS
& REFERENCE https://qz.com/338767/the-man-who-brought-us-the-lithium-ion-battery-at-57-has-an-idea-for-a-new-one-at-92/ https://qz.com/338767/the-man-who-brought-us-the-lithium-ion-battery-at-57-has-an-idea-for-a-new-one-at-92/ https://www.theguardian.com/politics/2021/jan/03/uk-carmakers-have-three-years-to-source-local-electric-car-batteries https://www.theguardian.com/politics/2021/jan/03/uk-carmakers-have-three-years-to-source-local-electric-car-batteries
Please
use our A-Z
INDEX to navigate this site
This
website is provided on a free basis as a public information service.
copyright © Climate Change Trust 2022. Solar
Studios, BN271RF, United Kingdom.
|







