|
SMART
SERVICE STATIONS - The same service station that provides freshly charged
hydrogen batteries, can also supply methanol batteries and/or ammonia
batteries. SmartNet is future proofing for investors such as energy
providers and grid operators, without resorting to gas
holders. Why put in load levelling stations that only have one
function? We hope this is a topic of discussion at the forthcoming UN
COP 27 in Egypt in 2022.
Ammonia has a higher energy density than liquid hydrogen (105kg hydrogen per cubic metre, compared to 71kg in liquid hydrogen). It
is therefore a potential candidate for hydrogen batteries, using the
Universal Cartridge system as part of the SmartNet system, to future proof
against technology changes and upgrades - as EVs come of age. There
are though as yet, technical obstacles to overcome. But with dedicated
R&D, it may be possible for ammonia, like methanol,
to become a fuel of choice, where in theory it could deliver hydrogen more
cheaply than as a compressed or cryogenic liquid. With
so many competing hydrogen based fuels, lithium and solid state batteries
all being developed in the zero emission gold-rush, automobile and truck
producers might increase the versatility of their range, protecting products
from obsolescence, as breakthroughs occur from the veritable stampede of
formats - shaking confidence in the market.
MORE ABOUT AMMONIA Ammonia
is less flammable and leakage is easier to detect by the smell (hydrogen is odourless).
Unfortnately, ammonia still needs to be stored as low temperatures
-33˚C rather than super-cooled -253˚C for liquid hydrogen.
Due to ammonia’s widespread use in farming, the distribution infrastructure already exists. Globally, around 180 million metric tonnes of ammonia are produced annually, and 120 ports are already equipped with ammonia terminals.
Ammonia (NH3), the colorless, pungent gas, is an excellent source of hydrogen in its liquid form, containing twice as much hydrogen as liquid hydrogen by volume. Liquid ammonia can be stored in large tanks at room temperature and is safer than propane and as safe as gasoline. More than 200 million tons of ammonia are produced each year and distributed globally via pipelines, tankers & trucks, making ammonia readily available and cheap.
When considering carbon-neutral pathways starting from sun, wind, air, and water,
a team from Delaware University show that ammonia has the lowest source-to-tank cost.
They focused on the direct ammonia fuel cell as the key technological barrier to realizing the economic benefits of ammonia.
Demonstrating how a high peak power density of 135 mW cm−2 can be achieved using ammonia-tolerant catalysts, high-stability hydroxide exchange membranes, and optimized operating conditions. 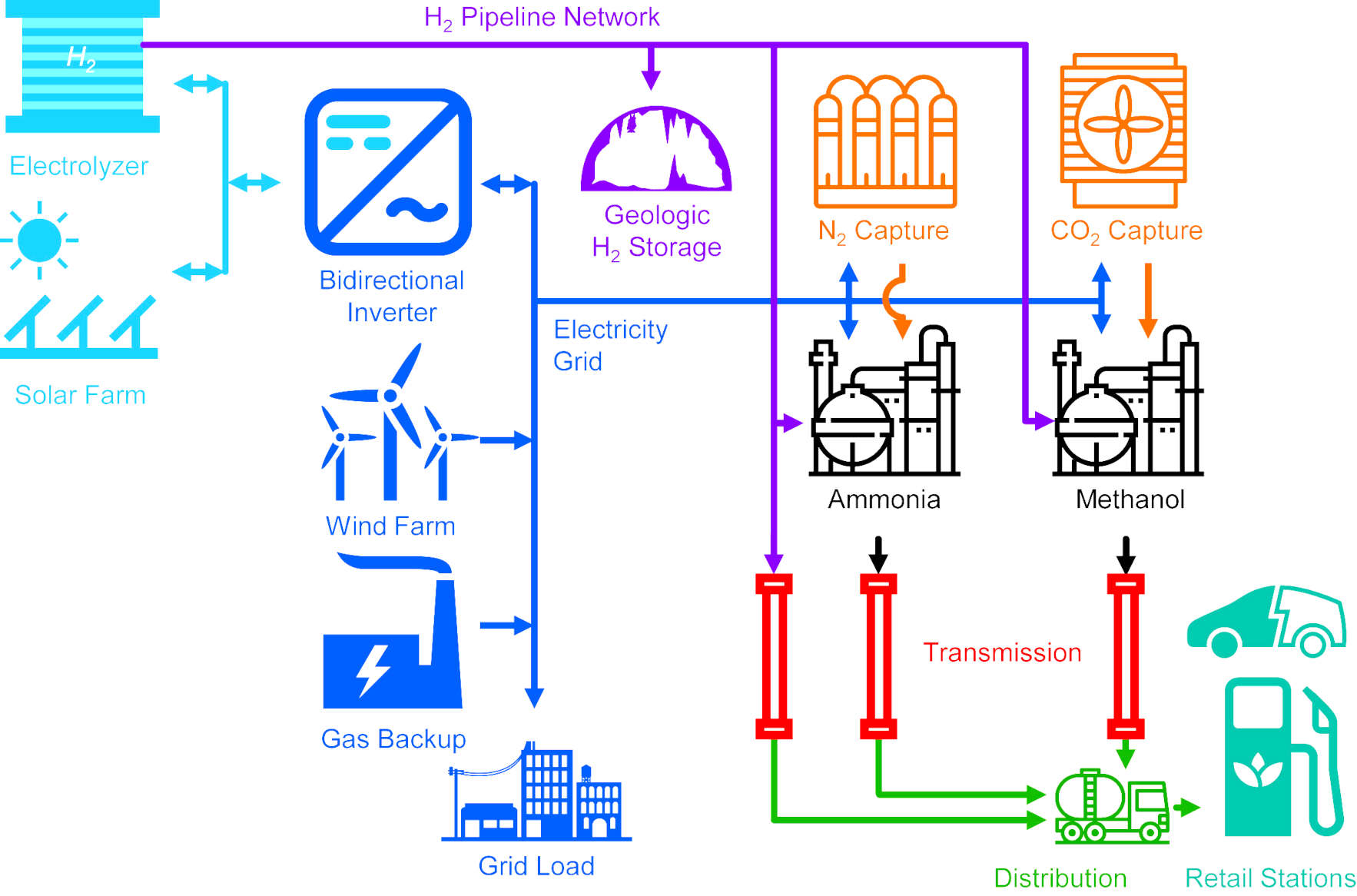
An Efficient Direct Ammonia Fuel Cell for Affordable Carbon-Neutral Transportation
Yun Zhao - Brian P.Setzler - Junhua Wang - Jared Nash - Teng Wang - Bingjun Xu - Yushan Yan
https://www.cell.com/joule/fulltext/S2542-4351(19)30321-6 https://www.cell.com/joule/fulltext/S2542-4351(19)30321-6 https://www.sciencedirect.com/science/article/pii/S2542435119303216 https://www.sciencedirect.com/science/article/pii/S2542435119303216
On the basis of hydrogen and carbon dioxide inputs, process steps, and fuel vapor pressure, hydrogen, ammonia, and methanol are identified as the most cost-effective candidates among hydrogen-, nitrogen-, and carbon-based fuels, respectively. With similar inputs, methanol as a liquid is favored cost-wise over dimethyl ether as a liquefied gas. Alternative nitrogen-based fuels such as hydrazine are inherently more expensive than ammonia, as they are produced from ammonia at low energy efficiencies. Urea is non-toxic, easy to produce from
ammonia, and highly soluble in water, but loses the advantages of other nitrogen-based fuels due to a high carbon dioxide content.
The delivered energy costs of carbon-neutral hydrogen, ammonia, and methanol transportation fuels are shown in Figure 3. On a source-to-tank basis, ammonia costs ($4.50 GGE−1) are 31% lower than hydrogen ($6.55 GGE−1) and 18% lower than methanol ($5.46 GGE−1). The importance of the key production metrics outlined in Table 1 are validated by the results in Figure 3. The high cost of hydrogen is due to high transmission, distribution, and dispensation costs (totaling $4.16 GGE−1) as a compressed gas. The cost penalty of methanol over ammonia is due to the high cost of separating carbon dioxide from air ($195 t−1), a factor that applies similarly or greater to all other carbon-containing fuels.
In summary, the economics of carbon-neutral synthetic fuels were analyzed, and results are described first, showing that ammonia has the lowest source-to-tank cost among fuels that can be produced from water, air, and renewable electricity. The suitability of ammonia for transportation depends on the performance of ammonia fueled power sources, including the direct ammonia fuel cell (DAFC). Second, two key factors are identified for achieving high DAFC performance: the use of a PGM-free cathode catalyst of zero AOR activity and a HEM which is highly stable at 80°C or higher. With optimized operating conditions, a peak power density of 135 mW cm−2 was demonstrated.
Substantial improvements in performance are still needed for DAFCs to become a practical power source for transportation applications. Desirable developments include minimized crossing of ammonia and controlled flow of water beyond the anode, requiring improvement of MEA structures and HEMs of low ammonia permeability maintaining good anionic conductivity. AOR catalysts with improved mass activity are needed to lower costs and increase efficiency. At the system level, recycling of ammonia from the anode exhaust and reduction of trace ammonia emissions are needed to create a practical transportation power source. Finally, stable DAFC operation over at least several days would contribute substantially to building of confidence in the DAFCs as potential power source for transportation.
https://techxplore.com/news/2021-03-world-high-temperature-ammonia-powered-fuel-cell.html
https://techxplore.com/news/2021-03-world-high-temperature-ammonia-powered-fuel-cell.html 
WORLD'S FIRST HIGH TEMPERATURE AMMONIA FUEL CELL FOR SHIPPING
As scientists around the world test new propulsion methods capable of replacing fuel oil in ships, Fraunhofer researchers are working as part of an international consortium to develop ammonia-based fuel cells. When used as fuel for ships with electric engines, ammonia is as eco-friendly as hydrogen, but easier and safer to handle.
At present, hydrogen is the primary focus in the area of sustainable energy: there are plans in place to use hydrogen to fuel buses, commercial vehicles, and even cars. However, the Fraunhofer Institute for Microengineering and Microsystems IMM in Mainz is working on another promising possibility. As part of the ShipFC project, the Fraunhofer Institute is collaborating with 13 European consortium partners to develop the world's first ammonia-based fuel cell for shipping. Fraunhofer's researchers are responsible for developing the catalytic converter, which prevents the production of emissions that would harm the climate.
Maritime transport is a major contributor to greenhouse gas emissions. According to information provided by the German Environment Agency (UBA), maritime transport on the world's oceans is currently responsible for approximately 2.6 percent of global CO2 emissions. In 2015, about 932 million tons of CO2 were emitted, and that figure increases every year. Clearly, countermeasures are urgently needed.
The ShipFC project is intended to prove that the new emission-free propulsion technology works safely, reliably, and smoothly, even in large ships and on long voyages. The project is being coordinated by NCE Maritime Cleantech from Norway, an organization that aims to develop eco-friendly technologies in the maritime sector.
The advantages of ammonia
Ammonia is primarily known for its use as a fertilizer in the agricultural sector. However, it can also function as a high-quality energy carrier. Prof. Gunther Kolb, director of the energy division and deputy institute director at the IMM, explains: "Ammonia has significant advantages over hydrogen. Hydrogen has to be stored at -253 degrees Celsius as a liquid, or at pressures of around 700 bar as a gas. Liquid ammonia can be stored at a reasonable temperature of -33 degrees Celsius at standard pressure, and +20 degrees Celsius at 9 bar. That makes storing and transporting this energy carrier considerably easier and more straightforward."
The process for generating electricity from ammonia functions in a similar way to hydrogen-based power plants. First, ammonia (NH3) is fed into a fission reactor, where it is split into nitrogen (N2) and hydrogen (H2). 75 percent of the gas consists of hydrogen. A small amount of ammonia (NH3, 100 ppm) is not converted and left over in the gas stream.
Second, nitrogen and hydrogen are fed into the fuel cell, and air is introduced, allowing the hydrogen to burn and form water. This produces electrical energy. However, the hydrogen isn't fully converted in the fuel cell. Around 12 percent of the hydrogen and some residual ammonia leave the fuel cell uncombusted. This residue is then fed into the catalytic converter developed by Fraunhofer IMM. Here, air is introduced and the residue comes into contact with a corrugated metal foil coated with a powdered layer of catalytic particles that contain platinum. This triggers a chemical reaction. Ultimately, the only end products are water and nitrogen. An optimal reaction process will not even produce environmentally harmful nitrogen oxides.
The research team at the IMM are also developing the reactor that contains the catalyst, which functions passively. The reactor controls the temperature and the gas flow. For example, it preheats the catalyst before the engines are even started, as it is less efficient when cold. "The temperature of the gases that flow through the catalytic converter should probably be around 500 degrees Celsius for the waste gas purification process to be as efficient as possible," explains Kolb.
Fraunhofer IMM researchers have decades of experience in developing reactors including catalysts for a wide variety of applications in the transport and mobility sector. The Mainz-based institute has nine test rigs, but purifying waste gas from ammonia fuel cells with a capacity of 2 megawatts is still a technological challenge. "We have to develop our existing ammonia-powered fuel cell technology further, and the catalytic converter for a ship is obviously much bigger than that of a normal car," says Kolb.
The status of the project
The IMM team is planning to complete an initial, small prototype by the end of 2021, to be followed by an actual-size prototype by the end of 2022. In the second half of 2023, the first ship with an ammonia-powered fuel cell will put out to
sea - the Viking Energy, a supply vessel owned by the Norwegian shipping company Eidesvik. After that, other types of vessels, such as cargo ships, will be equipped with ammonia-powered fuel cells.
Ammonia's potential for the future
The ammonia is provided by YARA, a partner in the ShipFC consortium. The chemical company currently produces one third of the ammonia used worldwide. The ShipFC project uses "green" ammonia, i.e. ammonia produced from renewable energy sources.
ShipFC is opening up major opportunities for a previously undervalued energy carrier. IMM researcher Gunther Kolb elaborates: "We see ammonia not as a direct competitor to hydrogen, but rather as an additional option in the area of sustainable energy. With its storage advantages, this environmentally friendly technology for power generation certainly has a role to play. Using it in ships is just the beginning."
Ammonia's potential has also been recognized at a political level, with the European Union providing 10 million euros in financial support for the ShipFC project.
https://fuelcellsworks.com/news/ammonia-for-fuel-cells/ https://fuelcellsworks.com/news/ammonia-for-fuel-cells/ 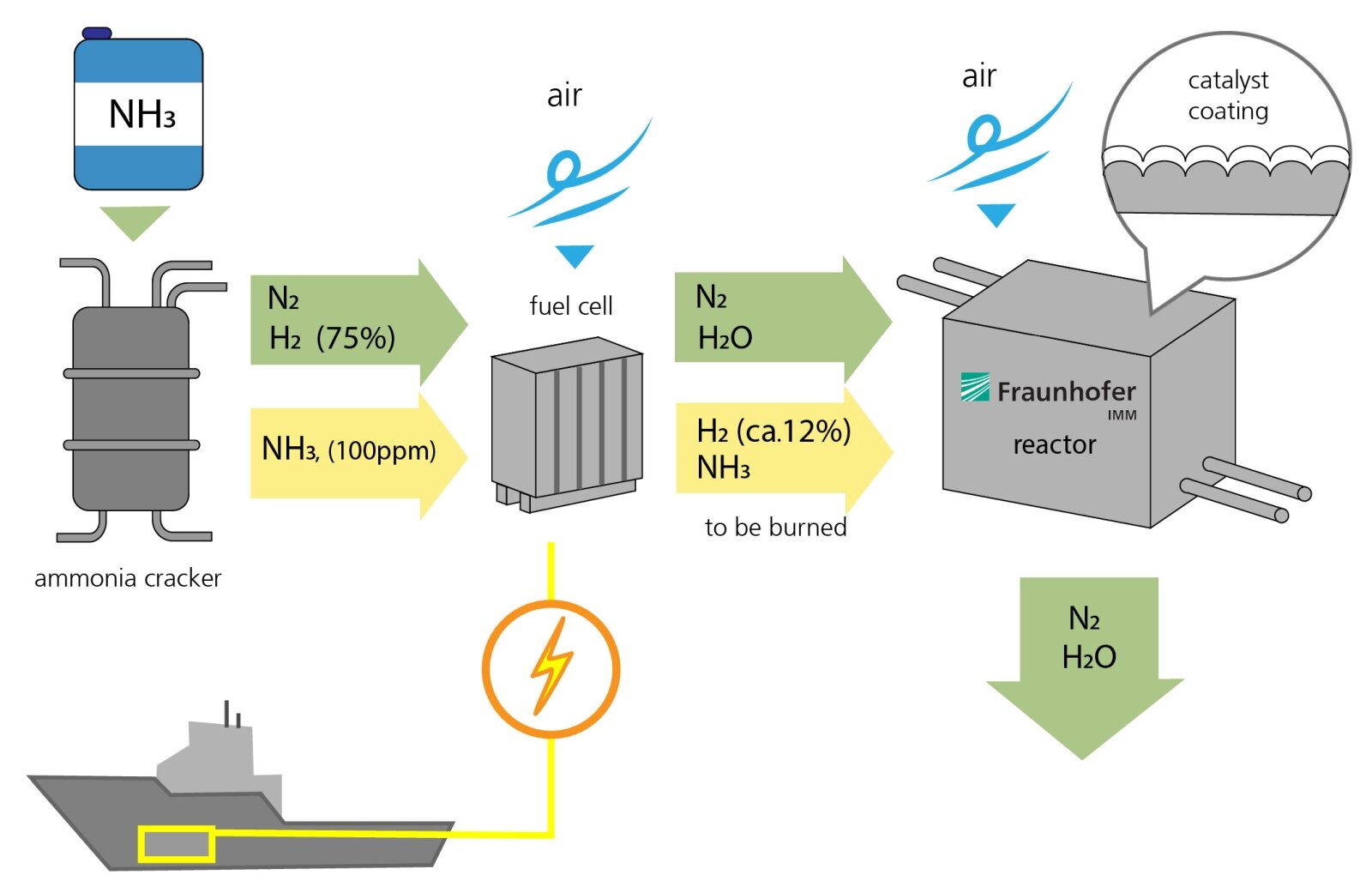
UNIVERSITY OF DELAWARE
ENGINEERING TEAM PUSHES FOR COST EFFECTIVE FUEL CELL TECHNOLOGY 16 AUGUST 2019
Fuel cells are pollution-free power sources that convert chemical energy to electricity with high efficiency and zero emissions. Fuel cell cars, trucks, and buses would allow people to travel long distances with convenient refueling and less of a carbon footprint.
Researchers at the University of Delaware are working on technology to make fuel cells cheaper and more powerful so that fuel cell vehicles can be a viable option for all someday. Traditional fuel cell research involves hydrogen fuel cells, but the UD researchers are engineering fuel cells that utilize ammonia instead.
In a new analysis published in the journal Joule, a team of engineers at the Center for Catalytic Science and Technology found that among fuels produced from renewable energy, ammonia has the lowest cost per equivalent gallon of
gasoline.
“As a nitrogen-based liquid fuel, ammonia is cheaper to store and distribute than hydrogen and avoids the carbon dioxide emissions of other liquid fuels, which are expensive to capture” said Brian Setzler, one of the lead authors and a postdoctoral associate at UD.
The challenges, however, are that ammonia does not work in a proton exchange membrane fuel cell; and that ammonia is more difficult to oxidize than hydrogen, which causes ammonia fuel cells to produce less power than hydrogen fuel cells. The team solved the first problem by using hydroxide exchange membrane fuel cells that have been studied for over a decade in the lab of Yushan Yan, a Distinguished Engineering Professor at UD. Assisted by a $2.5 million grant from the REFUEL program of the Advanced Research Projects Agency-Energy (ARPA-E) in the U.S. Department of Energy, the UD team engineered a fuel cell membrane that can operate at higher temperatures to speed up ammonia oxidation. They also identified catalysts that were not poisoned by ammonia.
“With these improvements, we have demonstrated a new direct ammonia fuel cell prototype with a peak power density of 135 milliwatts per square centimeter, which closes much of the performance gap compared to hydrogen,” said research associate Yun Zhao, the lead author of the paper who has been working on direct ammonia fuel cells since 2016.
The research team, all from UD’s Department of Chemical and Biomolecular Engineering, also includes research associate Junhua Wang, recent doctoral graduate Jared Nash, postdoctoral associate Teng Wang, assistant professor Bingjun Xu, and Distinguished Engineering Professor Yushan Yan.
https://www.sciencedirect.com/science/article/abs/pii/S2542435119303216 https://www.sciencedirect.com/science/article/abs/pii/S2542435119303216
Please
use our A-Z
INDEX to navigate this site
This
website is provided on a free basis as a public information service.
copyright © Climate Change Trust 2022. Solar
Studios, BN271RF, United Kingdom. SmartNet
™ is a registered trademark.
|



