|
ELECTROLYZERS
Please use our A-Z INDEX to navigate this site where page links may lead to other sites, or see HOME
|
|||
|
20 APRIL 2020
- The Japanese technology company Asahi Kasei is taking the next major step towards becoming a provider of large-scale alkaline-water electrolysis systems for the production of green hydrogen.
The key to making hydrogen the fuel of choice, is being able to produce large volumes at keen prices using renewable energy, that it is hoped will eventually be generated far in excess of the capacity to use on national grids.
To make this possible, electrolyzers need to be both efficient and cost effective.
Hydrogen is the only scalable technology capable of competitively meeting the huge needs of heavy-duty transport, which amount to hundreds or even thousands of kilograms of hydrogen every day.
Hydrogen is a a clean, alternative fuel, whose price at the pump competes with that of diesel, where bigger scale, lowers costs, in that the scaling up and industrialization of hydrogen stations will make it possible to bring about a drastic reduction in purchasing costs and the democratization of hydrogen mobility.
See World Electrolysis Congress Amsterdam, 13th December 2021 and 8th February Radisson, Hamburg 2022
| |||
|
|
| ||
|
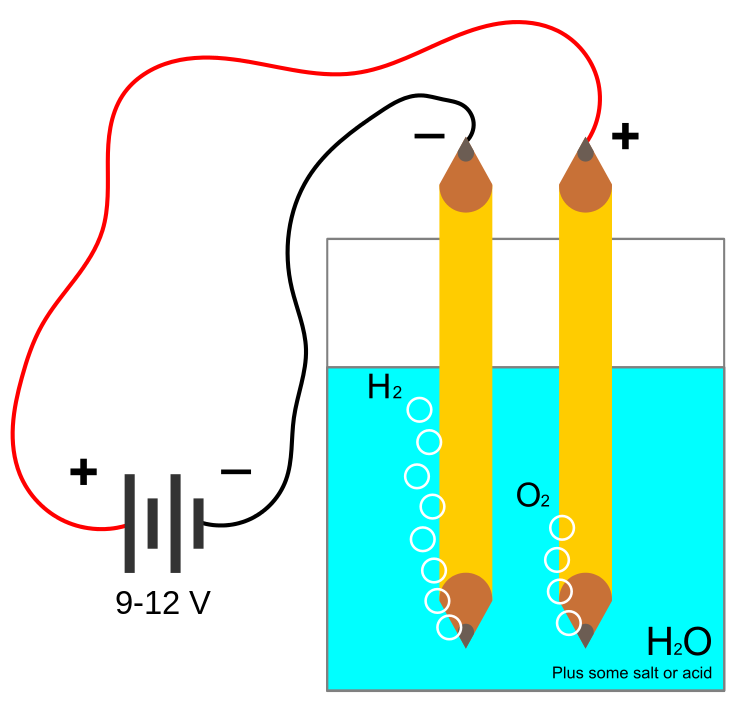
WHAT IS ELECTROLYSIS?
Electrolysis is the process by which ionic substances are decomposed (broken down) into simpler substances when an electric current is passed through them.
Efficiency of modern hydrogen generators is measured by energy consumed per standard volume of hydrogen (MJ/m3), assuming standard temperature and pressure of the H2. The lower the energy used by a generator, the higher its efficiency would be; a 100%-efficient electrolyser would consume 39.4 kilowatt-hours per kilogram (142 MJ/kg) of hydrogen, 12,749 joules per litre (12.75 MJ/m3). Practical electrolysis (using a rotating electrolyser at 15 bar pressure) may consume 50 kW⋅h/kg (180 MJ/kg), and a further 15 kW⋅h (54 MJ) if the hydrogen is compressed for use in hydrogen cars.
Considering the industrial production of hydrogen, and using current best processes for water electrolysis (PEM or alkaline electrolysis) which have an effective electrical efficiency of 70–80%, producing 1 kg of hydrogen (which has a specific energy of 143 MJ/kg) requires 50–55 kW⋅h (180–200 MJ) of electricity.
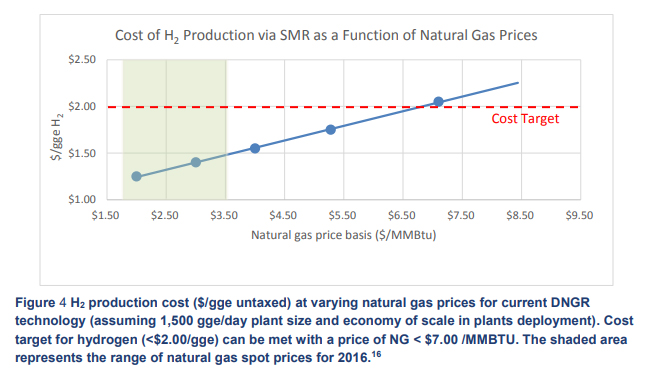
At an electricity cost of $0.06/kW·h, as set out in the US Department of Energy hydrogen production targets for 2015, the hydrogen cost is $3/kg. With the range of natural gas prices from 2016 as shown in the graph (Hydrogen Production Tech Team Roadmap, November 2017) putting the cost of steam-methane-reformed (SMR) hydrogen at between $1.20 and $1.50, the cost price of hydrogen via electrolysis is still over double 2015 DOE hydrogen target prices.
The US DOE target price for hydrogen in 2020 is $2.30/kg, requiring an electricity cost of $0.037/kW·h, which is achievable given 2018 PPA tenders for wind and solar in many regions. This puts the
$4/gasoline gallon equivalent (gge) H2 dispensed objective well within reach, and close to a slightly elevated natural gas production cost for SMR.
Sunfire’s pressurized Alkaline electrolyzer is optimal for applications without
(or with limited steam availability). With a system lifetime of at least 90,000 operating hours, the electrolyzer is our established solution for renewable hydrogen production.
LINKS & REFERENCE
https://mcphy.com/en/markets/mobility/ https://www.internationales-verkehrswesen.de/worlds-largest-single-stack-alkaline-water-electrolysis-system/
The Fuel Cells and Hydrogen Undertaking (FCH JU) are working to facilitate the market introduction of FCH technologies in Europe. They do this by implementing research and innovation (R&I) programmes in order to develop a portfolio of clean, efficient solutions that exploit the properties of hydrogen as an energy carrier and fuel cells as energy converters. They are not concerned with integration into a national infrastructure, just the enabling (seed) dots so that policy makers may join them.
Please use our A-Z INDEX to navigate this site
This website is provided on a free basis as a public information service. copyright © Climate Change Trust 2021. Solar Studios, BN271RF, United Kingdom.
| |||